Tailoring Polyamide Nanofiltration Membranes by Switching Charge of Nanocellulose Interlayers
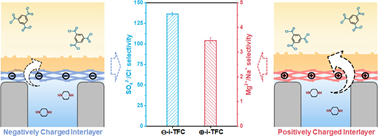
The interlayer strategy has emerged as an effective approach for modulating the interfacial polymerization process and improving the permeability and selectivity of polyamide membranes. However, the underlying mechanisms by which charged interlayers influence the interfacial polymerization process remain inadequately understood. In this study, we utilized two distinct charged cellulose nanofibers, namely, carboxylated cellulose (⊖-CNF) and quaternized cellulose (⊕-CNF), as interlayers to regulate the interfacial polymerization process. Through simulation results, isothermal titration calorimetry (ITC) and UV tests, we demonstrated that the ⊕-CNF interlayer, which possesses stronger hydration capability and better piperazine affinity, enhanced the diffusion of piperazine across the reaction interface compared with the ⊖-CNF interlayer. This led to an acceleration of the interfacial polymerization process and the formation of a denser membrane structure. Further investigation revealed that the charged interlayers significantly influenced the surface charging properties of the resulting nanofiltration membranes within a 30 nm range of electrostatic effects. Specifically, the ⊖-CNF interlayer conferred a higher negative charge to the membrane surface, while the ⊕-CNF interlayer endowed the membranes with a lower surface negative charge. Leveraging these differences, the ⊖-i-TFC membranes exhibited exceptional separation performance for divalent anions, achieving a SO42–/Cl– selectivity of 136. Conversely, the ⊕-i-TFC membrane demonstrated an enhanced separation of divalent cations, displaying a Mg2+/Na+ selectivity of 3.5. This study lays the groundwork for regulating the surface charging properties of polyamide membranes, offering potential advancements in nanofiltration applications.
.png)